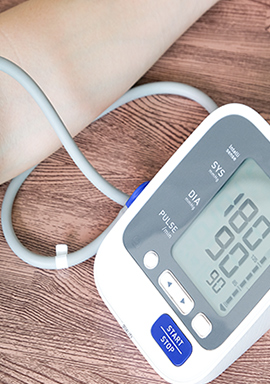
Blood Pressure Monitor Lawsuit Alleges Omron Platinum Readings Are ‘Wildly Inaccurate’
A class action alleges the maker of the Omron Platinum Upper Arm Blood Pressure Monitor has defrauded thousands of consumers in that blood pressure readings from the device are “wildly inaccurate.”