Class Action Lawsuit Claims Abbott Knowingly Sold Defective FreeStyle Libre 3 Glucose Monitors
Shroff v. Abbott Diabetes Care Inc. et al.
Filed: January 13, 2026 ◆§ 3:26cv351
A class action lawsuit alleges Abbott knowingly sold FreeStyle Libre 3 sensors that provided falsely low glucose readings.
California
A proposed class action lawsuit alleges that Abbott Laboratories has knowingly sold its FreeStyle Libre 3 and FreeStyle Libre 3 Plus continuous glucose monitoring systems that suffer from defects that hamper the accuracy and reliability of the sensors’ blood glucose readings.
Want to stay in the loop on class action lawsuits that matter to you? Sign up for ClassAction.org’s free weekly newsletter.
The 16-page defective product lawsuit contends that Abbott has concealed material safety information from individuals with Type 1 and Type 2 diabetes, who rely on glucose monitoring systems to provide accurate, real-time data on their blood glucose levels. The case says that, due to a manufacturing flaw on a certain production line in 2024, approximately three million FreeStyle Libre 3 sensors were made with a severe defect that could cause the devices to report “falsely low glucose readings,” even when a wearer’s blood glucose levels were already low, normal or elevated.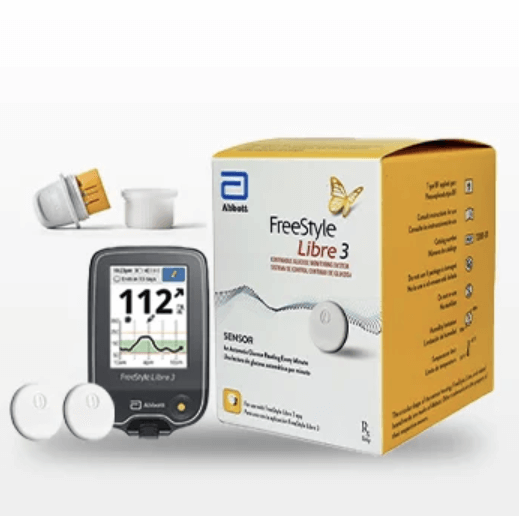
Related Reading: Class Action Case Alleges Dexcom Continuous Glucose Monitors Are ‘Adulterated,’ Sold Illegally
The FreeStyle Libre sensors are intended to replace traditional diabetes treatment and testing procedures, such as regular finger-pricking, and are implanted onto a user to provide 14 days of real-time blood glucose level reports, the complaint explains.
Abbott did not publicly disclose the glucose monitor defects or commence a broad corrective action until November 24, 2025, long after the defect already manifested on the market and in the wake of nearly 740 reports of serious injuries and seven deaths, the case says.
Days later, on December 2, the FDA issued an alert to warn the public about the defective FreeStyle Libre 3 sensors, with the agency advising that the devices be discontinued and replaced.
“Abbott’s delayed disclosure deprived consumers of important safety information necessary for their evaluation of whether to continue purchasing and using the Sensors,” the filing reads.
The complaint contends that Abbott has known of the glucose monitor defect from “its own internal testing and market surveillance,” and given the fact that the company recalled some of the sensors at issue in July 2024 due to incorrect high glucose readings. In September 2024, the suit continues, the FDA marked the measure as a Class I recall, the agency’s most serious designation, reserved for products that could reasonably cause serious adverse health effects or death.
The case maintains that the production line involved in the 2024 recall is the same as the one mentioned in the November 2025 recall.
“The shifting defects—going from dangerously high to dangerously low inaccuracies within 18 months—demonstrates a systemic failure in Abbott’s quality controls and a severe lack of effective Corrective and Preventive Actions required by the FDA,” the lawsuit emphasizes.
The plaintiff, a Florida resident, purchased the FreeStyle Libre 3 and the FreeStyle Libre Plus 3 Sensors multiple times to manage his diabetes. According to the lawsuit, the plaintiff experienced “dangerously inaccurate glucose readings” while using the FreeStyle Libre sensors in comparison to the levels ascertained through finger-pricking, before the consumer received a notice from CVS that the products he purchased were defective.
The Abbott glucose monitor class action lawsuit seeks to represent all individuals in the United States who purchased the FreeStyle Libre 3 or FreeStyle Libre 3 Plus Sensors during the applicable statute of limitations period.
Check out ClassAction.org’s free legal resources to learn how to file a class action lawsuit.
Video Game Addiction Lawsuits
If your child suffers from video game addiction — including Fortnite addiction or Roblox addiction — you may be able to take legal action. Gamers 18 to 22 may also qualify.
Learn more:Video Game Addiction Lawsuit
Depo-Provera Lawsuits
Anyone who received Depo-Provera or Depo-Provera SubQ injections and has been diagnosed with meningioma, a type of brain tumor, may be able to take legal action.
Read more: Depo-Provera Lawsuit
How Do I Join a Class Action Lawsuit?
Did you know there's usually nothing you need to do to join, sign up for, or add your name to new class action lawsuits when they're initially filed?
Read more here: How Do I Join a Class Action Lawsuit?
Stay Current
Sign Up For
Our Newsletter
New cases and investigations, settlement deadlines, and news straight to your inbox.
Before commenting, please review our comment policy.