Class Action Alleges Similasan Eye Drops Illegally Sold as Homeopathic Drugs Without FDA Approval [UPDATE]
Last Updated on May 7, 2025
Plowden v. Similasan Corp.
Filed: September 26, 2023 ◆§ 1:23-cv-02511
A proposed class action alleges Similasan has illegally marketed its eye drops as homeopathic drugs capable of curing a range of ophthalmic conditions.
Colorado
November 13, 2024 – Similasan Eye Drop Lawsuit Settled for $3.57 Million
Similasan has agreed to pay $3,575,000 to settle the proposed class action lawsuit detailed on this page.
Get all the details in ClassAction.org’s write-up about the Similasan eye drop settlement.
A proposed class action alleges Similasan has illegally marketed its eye drops as homeopathic drugs capable of curing a range of ophthalmic conditions.
Have you bought eye drops from any of the companies that received an FDA warning letter? Let us know here.
The 23-page case out of Colorado says that although Similasan touts that its eye drops can relieve eye aches, burning, redness, strain, itching, watering, blurred vision and dryness, the company has not received approval from the Food and Drug Administration (FDA) to market the products as homeopathic drugs or shown that the products are generally recognized as safe and effective.
As such, the Similasan eye drops at issue (listed below) are adulterated, unapproved drugs under federal law and cannot be sold to the public, the lawsuit says.
“Put simply,” the case asserts, “the Products should have never been marketed as homeopathic drugs.”
Similasan was one of several companies that received a warning letter from the FDA regarding their manufacture and marketing of “unapproved ophthalmic drugs” in violation of federal law. Per the complaint, the FDA found that the active ingredients in Similasan eye drops are not generally recognized as safe and effective, rendering the products “new drugs” that require FDA approval before they can be sold.
Had the Similasan eye drops gone through the FDA approval process, they may not have been approved as formulated, especially since some of the products contain silver sulfate as a preservative, the case states.
“The FDA has significant concerns regarding the safety of silver sulfate for use as an ophthalmic preservative. Long term use of medicinal compounds containing silver may cause argyria, which is a blueish-gray discoloration of the skin and eyes that is irreversible. Additionally, granular deposits of silver in the conjunctiva and cornea may cause decreased night vision. Accordingly, the FDA is concerned that Defendant’s use of silver sulfate as a preservative in ophthalmic products is inconsistent with [FDA regulations], under which ophthalmic preservatives should be ‘suitable and harmless.’”
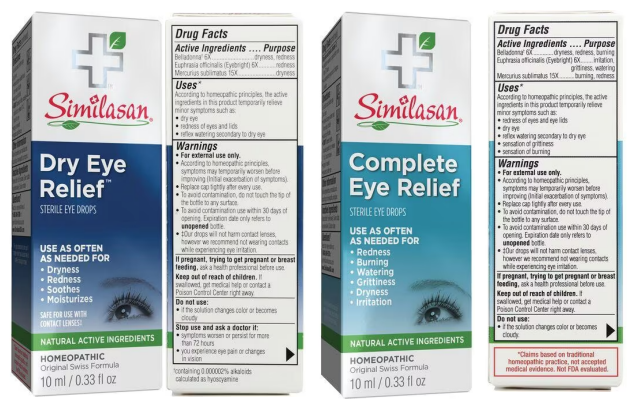 According to the suit, Similasan’s labeling of its eye drops purports that the products contain only “natural active ingredients,” and the company promises that the drops are different from traditional over-the-counter eye drops that use “chemicals to mask symptoms.” Further, Similasan says that the active ingredients in the eye drops “stimulate the body’s natural defenses, so you can feel better without harsh chemicals,” the filing relays.
According to the suit, Similasan’s labeling of its eye drops purports that the products contain only “natural active ingredients,” and the company promises that the drops are different from traditional over-the-counter eye drops that use “chemicals to mask symptoms.” Further, Similasan says that the active ingredients in the eye drops “stimulate the body’s natural defenses, so you can feel better without harsh chemicals,” the filing relays.
Want to stay in the loop on class actions that matter to you? Sign up for ClassAction.org’s free weekly newsletter here.
By labeling and marketing the eye drops as containing only homeopathic ingredients, Similasan warrants that the products were made in line with Current Good Manufacturing Practices (CGMPs), the case says.
However, the FDA has found that Similasan’s eye drops are produced by a contract manufacturer who has demonstrated “significant violations” of current good manufacturing practices, the suit claims. The case stresses that the safety and manufacturing methods for the Similasan eye drops are of particular concern given that the products are meant to be administered into the eyes, and as such “pose a greater risk of harm to users.”
“State consumer protection statutes and warranty law do not allow Defendant to materially misrepresent its Products as homeopathic drugs when they cannot legally be sold as such,” the lawsuit charges. “Defendant also should not have marketed and distributed a product that was not produced under the minimum required manufacturing and safety standards.”
The Similasan eye drops at issue in the lawsuit include:
- Similasan Dry Eye Relief;
- Similasan Complete Eye Relief;
- Similasan Allergy Eye Relief;
- Similasan Kids Allergy Eye Relief;
- Similasan Red Eye Relief;
- Similasan Pink Eye Relief;
- Similasan Kids Pink Eye Relief;
- Similasan Aging Eye Relief;
- Similasan Computer Eye Relief;
- Similasan Stye Eye Relief;
- Similasan Pink Eye Nighttime Gel; and
- Similasan Dry Eye Nighttime Gel.
The case argues that a reasonable consumer would not have bought the Similasan eye drops had they known they were not approved drugs and fell short of minimum safety and manufacturing standards.
The lawsuit looks to cover all consumers in the United States who, during the fullest period allowed by law, bought any of the Similasan eye drops listed on this page for personal use and not for resale.
Have you bought eye drops from any of the companies that received an FDA warning letter? Let us know here.
Video Game Addiction Lawsuits
If your child suffers from video game addiction — including Fortnite addiction or Roblox addiction — you may be able to take legal action. Gamers 18 to 22 may also qualify.
Learn more:Video Game Addiction Lawsuit
Depo-Provera Lawsuits
Anyone who received Depo-Provera or Depo-Provera SubQ injections and has been diagnosed with meningioma, a type of brain tumor, may be able to take legal action.
Read more: Depo-Provera Lawsuit
How Do I Join a Class Action Lawsuit?
Did you know there's usually nothing you need to do to join, sign up for, or add your name to new class action lawsuits when they're initially filed?
Read more here: How Do I Join a Class Action Lawsuit?
Stay Current
Sign Up For
Our Newsletter
New cases and investigations, settlement deadlines, and news straight to your inbox.
Before commenting, please review our comment policy.